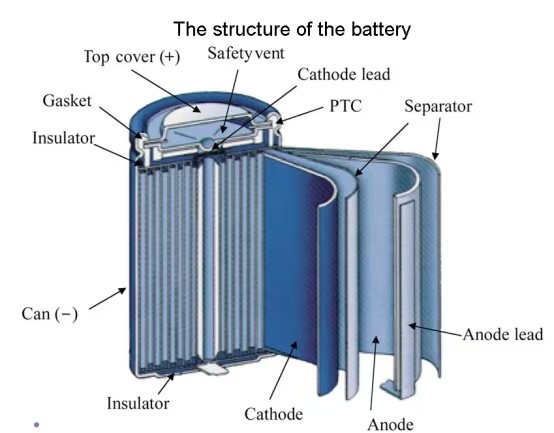
lithium battery is a type of battery that employs lithium’s chemical reactions to provide energy. It is a type of rechargeable battery (secondary battery) that is utilized in various devices, such as smartphones, laptops, electric vehicles, and electric tools. Here is a detailed description of the structure of a lithium battery:
Cathode (Positive electrode): The cathode is typically made up of lithium compounds like Lithium cobalt oxide (LiCoO2), Lithium nickel manganese cobalt oxide (LiNiMnCoO2), and Lithium iron phosphate (LiFePO4). During the charging process, lithium ions leave the cathode and move through the electrolyte to the anode. During the discharging process, the lithium ions move from the anode back to the cathode.
Anode (Negative electrode): The anode is mostly made of graphite, hard carbon, lithium titanate, or silicon and can absorb and release lithium ions. When the battery charges, lithium ions released from the cathode will migrate to the anode and embed in it. When the battery discharges, the lithium ions in the anode will be released and return to the cathode.
Electrolyte: The electrolyte serves as the electric conductor medium. It is the pathway through which ions migrate in the battery, facilitating rapid transmission of lithium ions between anode and cathode. Typically, this electrolyte is a mixture of lithium salts and an organic solvent.
In addition to these components, the lithium battery also includes a separator, which is situated between the anode and cathode. The separator prevents direct contact between anode and cathode, which could lead to a short circuit, while still allowing the passage of lithium ions. This separator is usually made from a microporous plastic film, having excellent ion conductivity and insulation.
Eventually, these components are all enclosed by a battery casing, which prevents moisture and oxygen from entering the battery. The casing has some venting holes to allow for pressure release in case the internal temperature of the battery rises too high for safety.